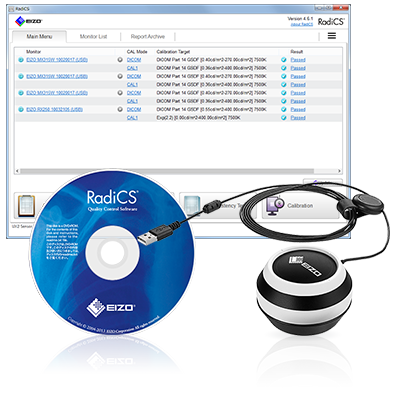
- RadiCS UX2

- 特性
- 规格
- 文件
- 显卡
- 质量控制软件和工具配件
Optimal Quality Control of Monitors in Your Hospital
Maintain Quality Control of Individual Monitors
Ensuring that the quality control of each client monitor complies with important medical standards, from calibration to acceptance and constancy tests to history and asset management, requires technical know-how and experience. EIZO offers software and sensors that makes quality control efficient and user-friendly.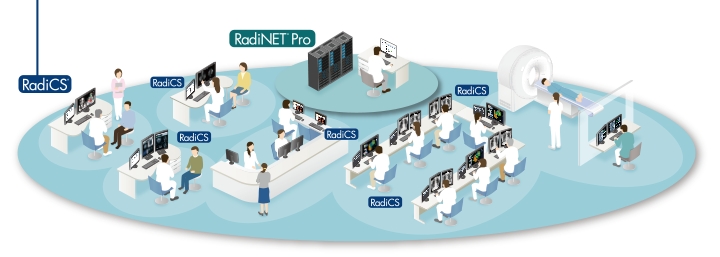
Improved User-Interface and Enhanced Operability
Graphical design and icons are arranged next to the text making it easy to comprehend the functions visually and intuitively. A compendium list also enables users to check the condition of monitors instantaneously. Furthermore, RadiCS simplifies operability such as gaining access to necessary information with just one click of a mouse.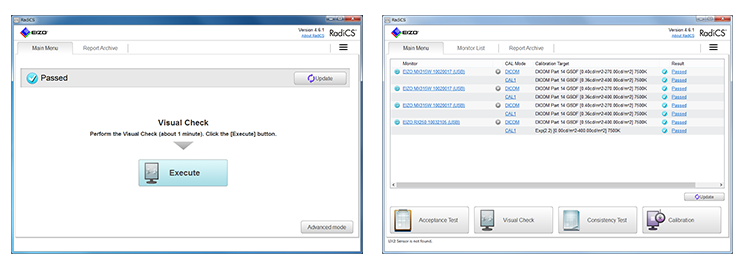
Acceptance and Constancy Testing in Easy Steps
RadiCS enables you to perform brightness, grayscale and uniformity checks that comply with AAPM TG18, DIN 6868-157, and other QC standards.
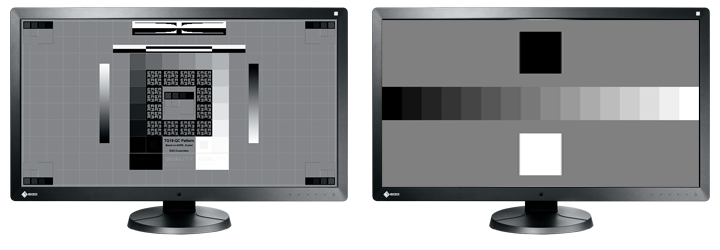
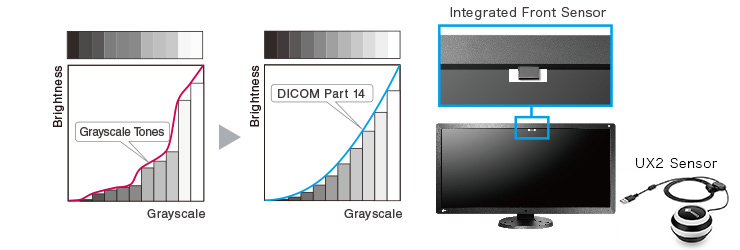
DICOM Part 14 Calibration
The built-in backlight sensor enables simplified calibration compliant with the DICOM Part 14 standard to correct the grayscale tones and brightness of the monitor. Furthermore, the use of an Integrated Front Sensor (IFS) or bundled UX2 Sensor enables higher calibration performance.
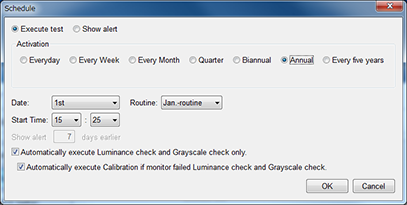
Flexible Schedule Setting
The timing of when to perform QC tasks such as daily tests or constancy tests can be set according to the needs of your institute. For example, when turning the PC on or just after a specific application is opened.
Other Features:
- Historical Management and Report Generator
- Continuous Asset Management
- Backlight Meter
- Distinct QC for All Modes
- Low-Power-Consumption with Backlight Saver
- Compatible with Non-RadiForce Monitors
"Assessment of Display Performance for Medical Imaging Systems" formulated by Task Group (TG) 18 of American Association of Physicists in Medicine.
  ACR-AAPM-SIIM "Practice Guideline for Determinants of Image Quality in Digital Mammography" ACR-AAPM-SIIM "Practice Guideline for Determinants of Image Quality in Digital Mammography"
  NYC Quality Assurance Guidelines for Primary Diagnostic Monitors NYC Quality Assurance Guidelines for Primary Diagnostic Monitors
"Recommended Standards for the Routine Performance Testing of Diagnostic X-ray Imaging Systems" formulated by the Institute of Physics and Engineering in Medicine in the UK.
"Image Quality Assurance in X-ray Diagnosis - Part 57: Acceptance testing for image display devices" formulated by the German Institute for Standardization (Deutsches Institut für Normung e.V).
"Guideline for implementing quality assurance of the X-ray systems for diagnostic and medical treatment purposes according to the chapter 16 and 17 of the X-ray Ordinance". This defines the details of the quality assurance of general X-ray systems obliged by the X-ray Ordinance.
|
| 兼容的操作系统 | Windows 10 Windows 8.1 Windows 8 Windows 7 / Windows 7 SP1 macOS Sierra (10.12) OS X El Capitan (10.11) |
|---|---|
| 显示功能 | DICOM Part 14 GSDF, CIE, 指数 (伽玛值), Log Linear, Linear,用户定义 |
| 接口 | USB, DDC, DDC/CI, RS232C |
| 语言 | 英语,德语,日语,中文,法文 |
| 包装内容 | RadiCS DVD-ROM (RadiCS, 使用手册), UX2 Sensor, Storage case, Adsorptive sheet for the replacement, Cleaning cloth, User's Manual |
质量控制软件及工具

- RadiCS 主要版本的软件

- 对于市场上其它显示器使用 RadiCS 的许可

 EUREF "European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis Fourth Edition"
EUREF "European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis Fourth Edition"
 IPEM Report 91
IPEM Report 91
 DIN 6868-157
DIN 6868-157

 ONR 195240-20: 2017
ONR 195240-20: 2017  JESRA X-0093*B-2017
JESRA X-0093*B-2017 "Quality Assurance (QA) Guideline for Medical Imaging Display Systems" formulated by Japan Industries Association of Radiological Systems (JIRA).
"Quality Assurance (QA) Guideline for Medical Imaging Display Systems" formulated by Japan Industries Association of Radiological Systems (JIRA).